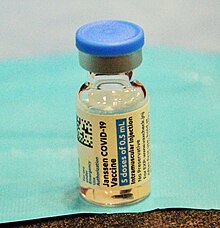
Vaksin COVID-19 Johnson & Johnson
Vaksin COVID-19 Johnson & Johnson ialah vaksin COVID-19 vektor virus adenovirus manusia[10] yang dibangunkan oleh Janssen Vaccines di Leiden, Belanda,[11] dan syarikat induknya di Belgium, Janssen Pharmaceuticals,[12] anak syarikat milik syarikat farmaseutikal Amerika Syarikat Johnson & Johnson (J&J).[13][14]
 | |
| Butiran vaksin | |
|---|---|
| Penyakit sasaran | SARS-CoV-2 |
| Jenis | viral |
| Data klinikal | |
| Nama dagang | Janssen COVID-19 Vaccine,[1][2] COVID-19 Vaccine Janssen |
| Nama lain | |
| Data lesen | |
| Kaedah pemberian | Intraotot |
| Kod ATC | |
| Status perundangan | |
| Status perundangan | |
| Pengecam | |
| DrugBank | |
| UNII | |
Vaksin tersebut mengandungi bahan asas adenovirus manusia yang diubahsuai untuk membuat gen untuk mencipta protein terhadap virus SARS-CoV-2 yang menyebabkan COVID-19. Vaksin tersebut hanya diwajibkan satu dos dan tidak perlu disimpan beku.
Rujukan sunting
- ^ a b "Janssen COVID-19 Vaccine- ad26.cov2.s injection, suspension". DailyMed. Dicapai pada 27 February 2021.
- ^ "Janssen COVID-19 Emergency Use Authorization (EUA) Official Website". Janssen. 28 February 2021. Dicapai pada 28 February 2021.
- ^ a b c d "A Randomized, Double-blind, Placebo-controlled Phase 3 Study to Assess the Efficacy and Safety of Ad26.COV2.S for the Prevention of SARS-CoV-2-mediated COVID-19 in Adults Aged 18 Years and Older ENSEMBLE Protocol VAC31518COV3001; Phase 3" (PDF). Janssen Vaccines & Prevention.
- ^ a b c d "A Randomized, Double-blind, Placebo-controlled Phase 3 Study to Assess the Efficacy and Safety of Ad26.COV2.S for the Prevention of SARS-CoV-2-mediated COVID-19 in Adults Aged 18 Years and Older ENSEMBLE 2 Protocol VAC31518COV3009; Phase 3" (PDF). Janssen Vaccines & Prevention.
- ^ "Janssen COVID-19 Vaccine monograph" (PDF). Janssen. 5 March 2021.
- ^ "FDA Issues Emergency Use Authorization for Third COVID-19 Vaccine". U.S. Food and Drug Administration (FDA) (Siaran akhbar). 27 February 2021. Dicapai pada 27 February 2021.
- ^ https://www.fda.gov/media/146303/download
- ^ "COVID-19 Vaccine Janssen EPAR". European Medicines Agency (EMA). 5 March 2021. Dicapai pada 16 March 2021.
- ^ "A Study of Ad26.COV2.S for the Prevention of SARS-CoV-2-Mediated COVID-19 in Adult Participants (ENSEMBLE)". ClinicalTrials.gov. Dicapai pada 30 January 2021.
- ^ "Leiden developed Covid-19 vaccine submitted to EMA for approval". 2021-02-16.
- ^ "Clinical trial COVID-19 vaccine candidate underway". Janssen Belgium (dalam bahasa Inggeris). Dicapai pada 2021-03-13.
- ^ "EMA recommends Johnson & Johnson Covid vaccine for approval; Developed in Leiden". NL Times.
- ^ Saltzman, Jonathan (12 March 2020). "Beth Israel is working with Johnson & Johnson on a coronavirus vaccine". The Boston Globe.
Pautan luar sunting
| Scholia mempunyai profil tentang Vaksin COVID-19 Johnson & Johnson (Q98655215). |